Apoptosis, or programmed cell death, is a critical process for maintaining tissue homeostasis and removing damaged or abnormal cells from the body. Dysregulation of apoptosis can contribute to various diseases, including cancer, autoimmune disorders, and neurodegenerative conditions. In recent years, targeting the anti-apoptotic protein MCL1 has emerged as a potential therapeutic strategy to restore apoptotic pathways. In this article, we will explore the MCL1 Targeted Library and its role in developing innovative therapies aimed at reestablishing apoptotic balance for improved disease management.
Understanding MCL1 and Apoptosis:
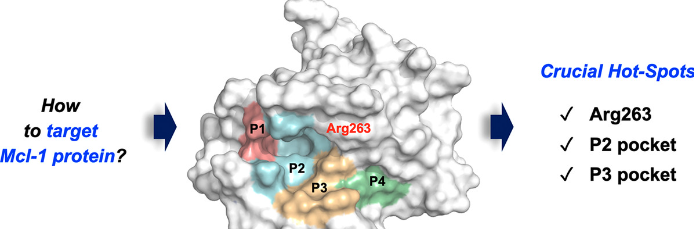
MCL1 is a member of the BCL-2 protein family, known for its role in inhibiting apoptosis by preventing the release of mitochondrial cytochrome c and activation of downstream caspases. MCL1 overexpression is frequently observed in various cancers and confers resistance to chemotherapy and targeted therapies. Inhibiting MCL1 represents a promising approach to tip the apoptotic balance in favor of cell death and sensitize cancer cells to treatment.
The MCL1 Targeted Library:
The MCL1 Targeted Library comprises a collection of small molecule inhibitors designed to selectively bind to and inhibit the activity of MCL1. These inhibitors are developed using computational modeling, structure-activity relationship analysis, and optimization techniques to enhance potency, selectivity, and drug-like properties. The MCL1 Targeted Library provides researchers with a valuable resource for screening and identifying potential lead compounds for further preclinical and clinical development.
Restoring Apoptosis in Cancer:
Targeting MCL1 offers a unique opportunity for restoring apoptotic pathways in cancer cells and overcoming treatment resistance. By inhibiting MCL1, it becomes possible to promote the release of pro-apoptotic factors from the mitochondria, leading to the activation of downstream caspases and induction of cell death. Numerous studies have demonstrated the potential of MCL1 inhibitors to sensitize cancer cells to chemotherapy, targeted therapies, and immunotherapy, offering a promising avenue for improved clinical outcomes.
Expanding Applications Beyond Cancer:
While the primary focus of the MCL1 Targeted Library lies in cancer therapeutics, there is growing evidence suggesting a role for MCL1 in other diseases as well. Inhibition of MCL1 has shown promise in autoimmune diseases, where aberrant apoptosis regulation contributes to autoimmunity. Additionally, targeting MCL1 may hold potential in neurodegenerative disorders, where neuronal apoptosis plays a significant role in disease progression. Further research is needed to fully explore these therapeutic opportunities.
Challenges and Future Directions:
Developing selective and potent MCL1 inhibitors presents several challenges. Designing inhibitors that specifically target MCL1 while avoiding cross-reactivity with other BCL-2 family members is crucial. Also, understanding the intricate regulation of MCL1 expression and the potential compensatory mechanisms that cancer cells may employ are areas that warrant further investigation. The MCL1 Targeted Library provides a foundation for future research and optimization efforts to address these challenges.
Conclusion:
The MCL1 Targeted Library represents an innovative and valuable resource for researchers and drug developers aiming to restore apoptotic pathways in various diseases. By selectively targeting MCL1, it becomes possible to overcome treatment resistance and sensitize cancer cells to therapy. The potential applications of MCL1 inhibitors extend beyond cancer to autoimmune disorders and neurodegenerative diseases, offering hope for improved disease management. As research in MCL1 inhibition continues to progress, the MCL1 Targeted Library plays a crucial role in advancing therapeutic interventions that restore apoptotic balance, ultimately leading to better patient outcomes.