Introduction:
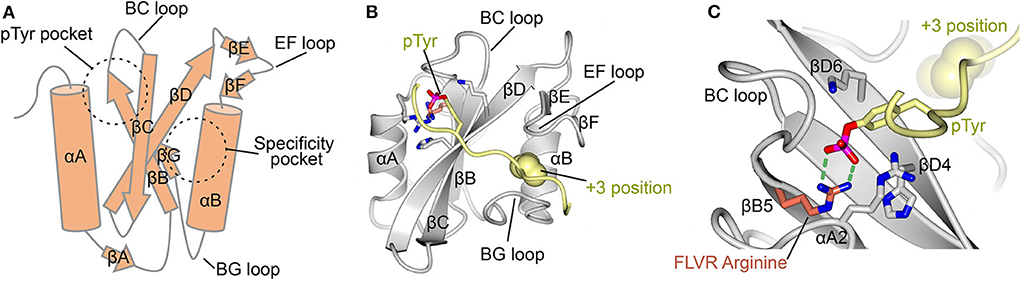
Cellular signaling plays a pivotal role in coordinating complex biological processes and maintaining cellular homeostasis. The Src Homology 2 (SH2) domain is a conserved protein module found in numerous signaling proteins involved in key cellular events, including growth, differentiation, and immune responses. The SH2 domain acts as a “molecular switchboard,” recognizing and binding to specific phosphotyrosine (pY) residues on target proteins, thus transmitting critical signals within the cell. In this article, we will delve into the world of the SH2 Library and its focus on developing SH2 domain binders for therapeutic applications.
Understanding SH2 Domain Binders:
The SH2 Library comprises a vast collection of small molecules designed to target and bind to the SH2 domains of specific signaling proteins. SH2 domain binders are compounds that mimic the pY-tyrosine interaction and effectively block or modulate specific signaling pathways. These small molecules offer an innovative platform for disrupting aberrant signaling events in diseases, demonstrating potential for therapeutic intervention.
Importance of SH2 Domain Binders:
SH2 domain binders provide a means to selectively interfere with signaling cascades that are dysregulated in various disease contexts. By binding to the SH2 domains of specific proteins, these compounds can disrupt protein-protein interactions critical for signal transduction, leading to the modulation or inhibition of aberrant signaling pathways contributing to disease pathogenesis. Additionally, SH2 domain binders can potentially restore normal signaling patterns or enhance desired signaling events, making them invaluable tools for therapeutic development.
Applications of SH2 Domain Binders:
The versatile nature of SH2 domain binders opens up avenues for therapeutic intervention in a variety of diseases. Dysregulation of signaling pathways involving receptor tyrosine kinases, non-receptor tyrosine kinases, or adaptor proteins, which frequently feature SH2 domains, has been implicated in cancer, immune disorders, and other diseases. The SH2 Library offers an extensive repertoire of compounds that can selectively target these signaling proteins, providing a means to disrupt specific pathways and potentially restore normal cellular function.
Challenges and Future Directions:
Development of effective SH2 domain binders faces several challenges. Achieving selectivity among SH2 domains, given their high sequence homology, is crucial to avoid off-target effects. The identification of appropriate lead compounds also demands a thorough understanding of the structural basis of SH2 domain-pY interactions. Furthermore, optimizing the pharmacokinetic and pharmacodynamic properties of SH2 domain binders is vital to ensure their efficacy and safety in vivo.
Conclusion:
The SH2 Library offers a promising avenue for therapeutic development by targeting signaling pathways involved in disease processes. SH2 domain binders hold great potential in selectively modulating or inhibiting aberrant signaling events, thus allowing for the development of innovative therapies for cancer, immune disorders, and other diseases. Continued research and optimization efforts in the field of SH2 domain binders will undoubtedly contribute to expanding our understanding of cellular signaling and provide new opportunities for precision medicine approaches in the future.