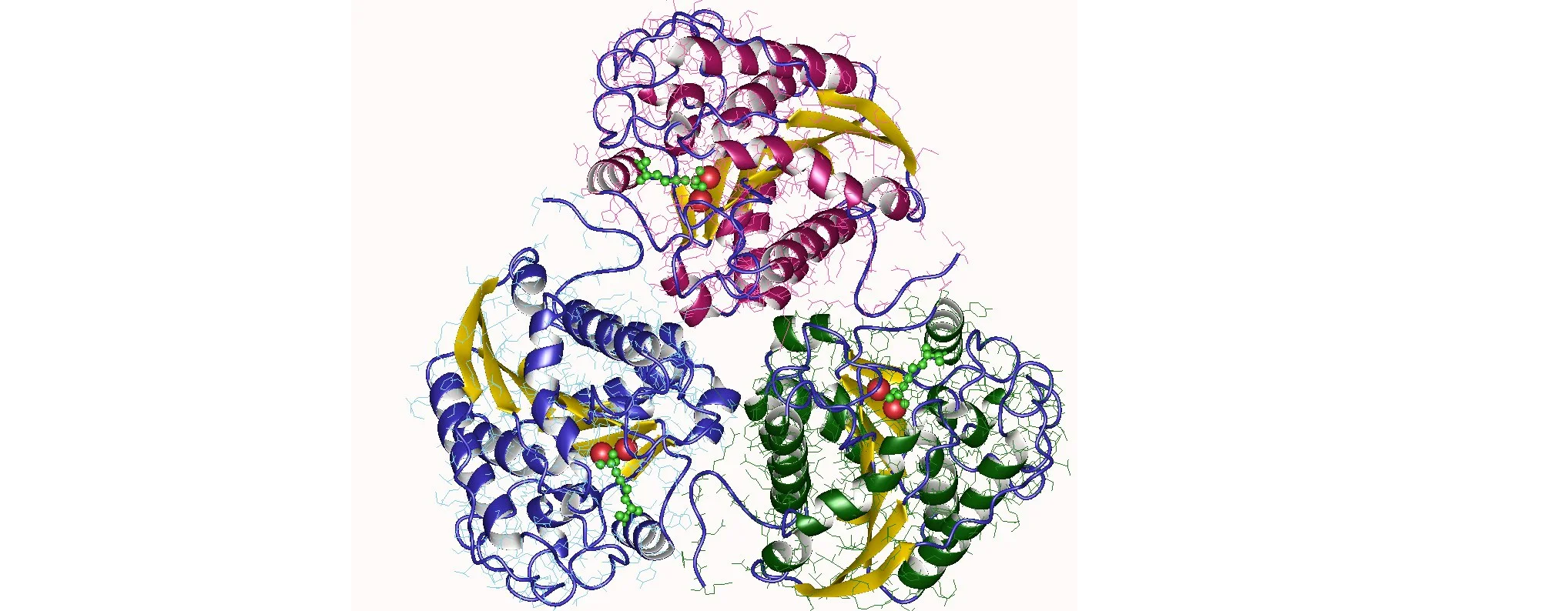
Arginase is a family of enzymes that plays a crucial role in the regulation of arginine metabolism. It catalyzes the hydrolysis of arginine to ornithine and urea, which are essential for various physiological processes. Overactivation of arginase has been implicated in several diseases, including cancer, cardiovascular diseases, and immune disorders. In recent years, targeting specific isoforms of arginase using the Arginase Targeted Library has emerged as a promising strategy for developing selective and effective inhibitors. In this article, we will explore the concept of isoform selective inhibition and the potential of the Arginase Targeted Library in developing novel therapeutics.
Understanding Arginase Isoforms:
There are two isoforms of arginase: arginase 1 (ARG1) and arginase 2 (ARG2). Although they share high sequence similarity, they differ in their tissue distribution and subcellular localization. ARG1 is primarily expressed in the liver and is involved in the urea cycle, while ARG2 is found in extrahepatic tissues, including the kidney, lung, and immune cells. Both isoforms contribute to arginine metabolism, but they may have different roles in disease pathogenesis.
The Arginase Targeted Library:
The Arginase Targeted Library comprises a collection of small molecules designed to selectively inhibit either ARG1 or ARG2. These inhibitors are developed using structure-based drug design, virtual screening, and medicinal chemistry approaches to enhance potency, selectivity, and pharmacokinetic properties. The Arginase Targeted Library provides researchers with valuable tools to investigate the isoform-specific roles of arginase and develop isoform selective inhibitors.
Isoform Selective Inhibition:
Isoform selective inhibition aims to specifically target one isoform of arginase while sparing the other. This approach provides two potential benefits: First, it allows for a better understanding of the individual isoform’s role in disease pathogenesis, facilitating the development of isoform-specific therapies. Second, isoform selective inhibitors may mitigate potential side effects associated with pan-arginase inhibition by sparing the isoform responsible for critical physiological functions.
Applications of Isoform Selective Inhibition:
Selective targeting of arginase isoforms holds promise in various diseases where arginase dysregulation plays a significant role. For instance, ARG1 is often upregulated in immune suppressive cells within the tumor microenvironment, leading to arginine depletion and immune evasion. Selective inhibition of ARG1 has been shown to restore immune function and potentiate immunotherapies in cancer. On the other hand, ARG2 has been associated with cardiovascular diseases and pulmonary hypertension, making it a potential therapeutic target in these conditions. The isoform selective inhibitors from the Arginase Targeted Library provide valuable tools to investigate these specific disease contexts.
Challenges and Future Directions:
Selective targeting of arginase isoforms presents several challenges. Arginase isoforms share high sequence similarity and possess similar active sites, making it difficult to develop inhibitors that selectively target one isoform over the other. Moreover, the isoform-specific roles of arginase in various diseases are still being elucidated, highlighting the need for further research to identify appropriate therapeutic opportunities.
Conclusion:
The Arginase Targeted Library offers a unique opportunity to explore isoform selective inhibition and develop potent and selective inhibitors against ARG1 and ARG2. By selectively targeting specific isoforms of arginase, it becomes possible to gain insights into their individual roles in disease pathogenesis and develop isoform-specific therapies. The isoform selective inhibitors from the Arginase Targeted Library hold promise in various diseases where arginase dysregulation plays a role, including cancer, cardiovascular diseases, and immune disorders.