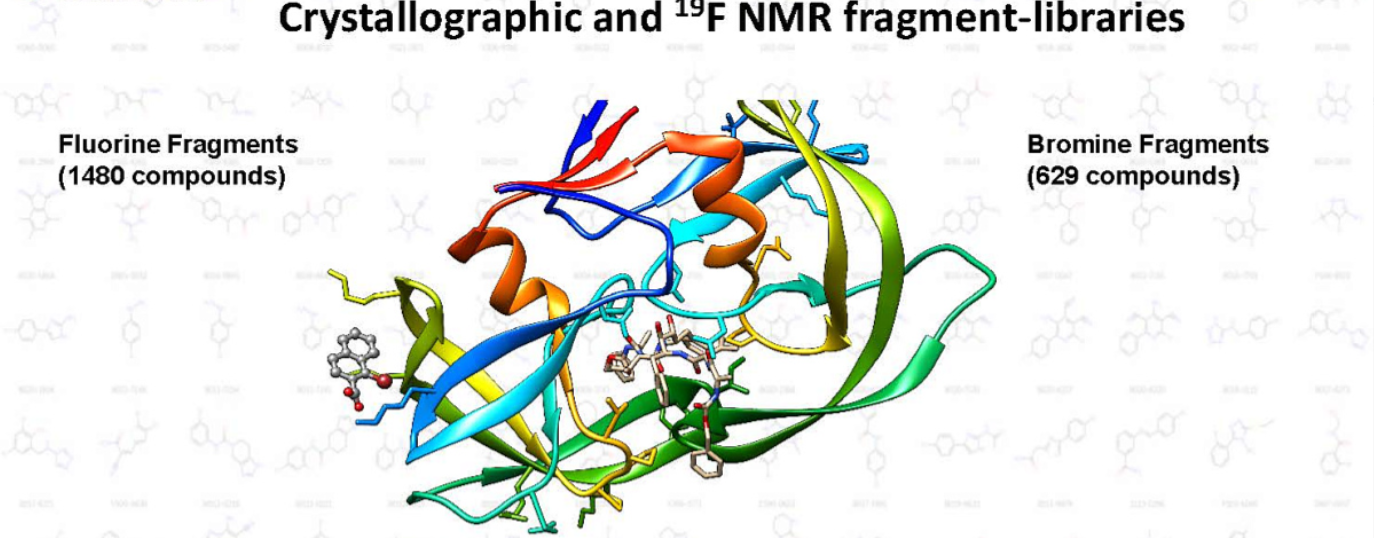
In the field of drug discovery, fragment-based approaches have gained tremendous importance in identifying novel lead compounds. Crystallographic and 19F NMR fragment libraries offer valuable insights into the structure and dynamics of small molecule-protein interactions. This article will delve into the significance of fluorine and bromine fragments in crystallographic and 19F NMR libraries, shedding light on how these libraries contribute to the discovery of potent and selective drug candidates.
Crystallographic Fragment Libraries:
Crystallographic fragment libraries leverage X-ray crystallography to determine high-resolution structures of protein fragment complexes. By utilizing a diverse repertoire of fragments, researchers can gain insights into the binding interactions and conformational changes that occur upon ligand binding. The inclusion of fluorine and bromine fragments in crystallographic libraries introduces additional chemical diversity, offering new avenues for structure-based drug design.
Fluorine fragments are particularly valuable tools in crystallography due to the unique physicochemical properties of fluorine atoms. Fluorine acts as a versatile probe, providing highly informative data on ligand-receptor interactions. The incorporation of fluorine atoms into fragments enhances their lipophilicity, improves their binding affinity, and imparts distinct structural features that can guide the optimization process. Fluorine can also act as a handle for radiofluorination, enabling the development of radiolabeled compounds for positron emission tomography (PET) imaging.
Bromine fragments, although less commonly used compared to fluorine fragments, offer complementary advantages in crystallography. Bromine atoms possess a larger size than fluorine, enabling them to interact with protein residues through van der Waals and halogen bonding interactions. These unique interactions can provide crucial insights into target binding pockets and the identification of hotspots for fragment optimization. Additionally, bromine atoms can be utilized for radiolabeling purposes, facilitating radiotracer development for imaging studies.
19F NMR Fragment Libraries:
19F NMR fragment libraries employ fluorine atoms as sensitive probes to study ligand-target interactions in solution. By monitoring changes in the NMR spectra upon fragment binding, researchers can assess binding affinities, stoichiometry, and structural dynamics. The inclusion of fluorine and bromine fragments expands the chemical diversity in 19F NMR libraries, enhancing the possibilities for investigating protein-ligand interactions in a solution-based setting.
Advantages and Applications:
The incorporation of fluorine and bromine fragments into crystallographic and 19F NMR libraries offers several advantages in drug discovery. These include:
Increased chemical diversity: Fluorine and bromine fragments expand the range of chemical functionalities, enabling exploration of a wider chemical space.
Enhanced target engagement: Fluorine and bromine atoms can form specific interactions with protein residues, offering opportunities for improved target engagement and selectivity.
Structural insights: The high-resolution structures obtained from crystallography provide critical information about ligand binding modes, guiding the design of optimized compounds.
Solution-based screening: 19F NMR libraries allow for efficient screening in solution, providing insights into binding kinetics, thermodynamics, and ligand-induced conformational changes.
Future Directions:
As crystallography and 19F NMR techniques continue to advance, so too will the utility of fluorine and bromine fragments in fragment-based drug discovery. Advances in fragment-based drug design and computational methods are paving the way for more efficient identification and optimization of lead compounds. Furthermore, the integration of these libraries with other screening approaches, such as virtual screening or fragment linking strategies, holds promise for accelerating the drug discovery process even further.
Conclusion:
Crystallographic and 19F NMR fragment libraries have the potential to unlock new chemical space and provide crucial insights into small molecule-protein interactions. With the inclusion of fluorine and bromine fragments, researchers gain access to powerful tools that enhance the understanding and optimization of drug candidates. Leveraging these libraries enables the discovery of potent and selective drugs, ultimately benefitting patients by providing innovative therapeutic interventions.