Cancer is a complex and heterogeneous disease that is characterized by uncontrolled cell growth and proliferation. In normal cells, a process called cellular senescence limits the number of times a cell can divide. However, cancer cells often bypass this senescence checkpoint through the activation of telomerase, an enzyme that maintains the length of telomeres, the protective ends of chromosomes. The human telomerase reverse transcriptase (hTERT) subunit is a catalytic component of telomerase and is overexpressed in the majority of cancer cells, making it an attractive target for cancer therapy. To specifically target hTERT and exploit its role in cancer development and progression, scientists have developed the hTERT-Targeted Library – a collection of small molecules designed to selectively inhibit the activity of hTERT and disrupt telomerase function.
The development of the hTERT-Targeted Library involves several key steps:
Target identification and validation:
The hTERT subunit of telomerase is identified as a therapeutic target through investigations into the mechanisms of telomere maintenance in cancer cells.
Extensive research and validation studies are conducted to confirm the overexpression of hTERT in various cancer types, its association with telomerase activity, and its role in promoting cancer cell survival and immortality.
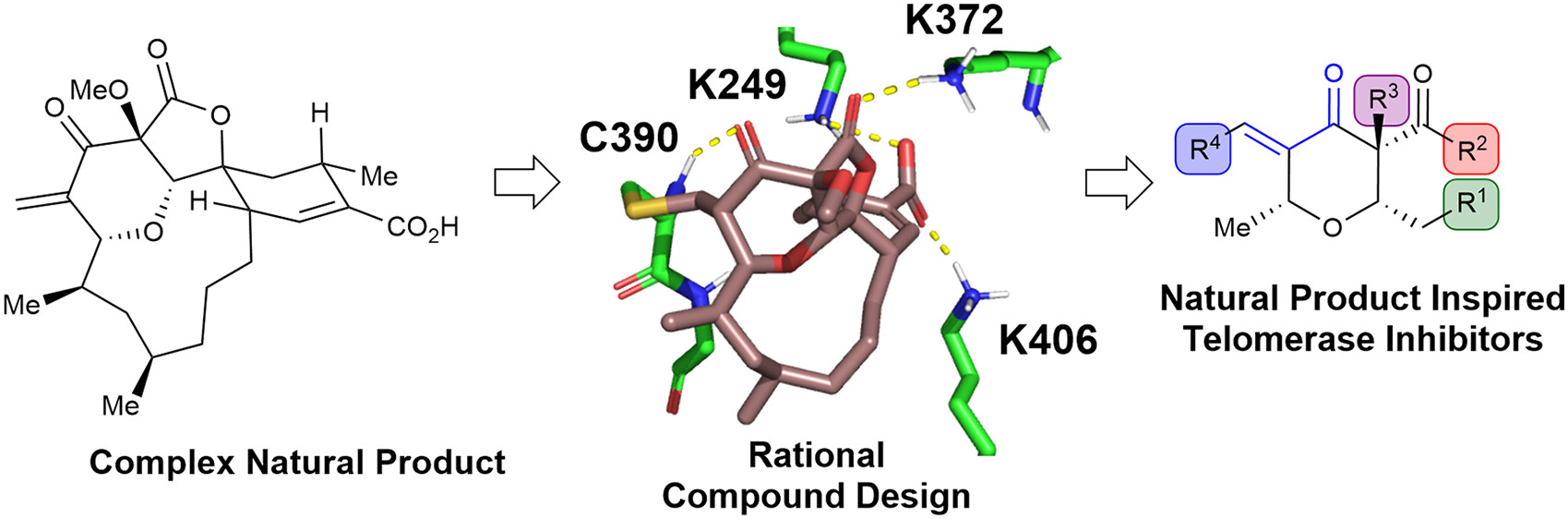
Rational drug design and virtual screening:
Structural and functional studies of hTERT provide insights into its three-dimensional structure and active site.
Computational techniques, such as molecular docking, structure-based drug design, and virtual screening, are employed to identify or design small molecules with potential binding affinity and specificity for the active site of hTERT.
Optimization and selectivity:
Initial hits or lead compounds identified from virtual screening undergo optimization processes to improve their potency, bioavailability, and drug-like properties.
Medicinal chemistry and structure-activity relationship (SAR) studies are conducted to fine-tune the chemical structure of the compounds and enhance their selectivity for hTERT.
High-throughput screening (HTS):
HTS approaches are utilized to efficiently screen a large number of compounds from the hTERT-Targeted Library for their ability to inhibit hTERT activity or disrupt telomerase function.
Assays measuring telomerase activity or telomere elongation can help identify potent and selective hTERT inhibitors.
Cellular and in vivo validation:
Promising hTERT inhibitors identified from HTS require further validation in cellular models and animal studies.
Cellular assays can assess the impact of hTERT inhibition on telomere length, cell viability, proliferation, and senescence in cancer cells.
Animal models provide insights into the efficacy, pharmacokinetics, toxicity, and potential side effects of hTERT inhibitors in vivo.
Combination therapies and personalized medicine:
hTERT inhibitors can be explored as monotherapies or in combination with other anticancer agents to enhance treatment outcomes.
Personalized medicine approaches, such as identifying specific cancer types or patient subsets with high hTERT expression, can help tailor treatment strategies and improve therapeutic efficacy.
The development of the hTERT-Targeted Library opens up new possibilities for cancer therapy by specifically targeting telomerase and disrupting telomere maintenance in cancer cells. By inhibiting hTERT activity, these compounds aim to induce telomere shortening, cellular senescence, and ultimately, the selective death of cancer cells without affecting normal cells. Additionally, targeting hTERT may overcome the limitations of traditional cancer treatments, such as chemotherapy and radiation, which often cause systemic toxicity and damage to healthy tissues.
It is important to note that translating the discoveries from the hTERT-Targeted Library into clinically approved drugs requires extensive preclinical and clinical studies to ensure safety, efficacy, and regulatory approval. Furthermore, the complexity of cancer and the heterogeneity of tumors necessitate further research and refinement of therapeutic strategies to circumvent potential resistance mechanisms and optimize treatment outcomes.
In conclusion, the hTERT-Targeted Library represents a significant advancement in the development of cancer therapeutics. By selectively targeting hTERT and disrupting telomerase function, researchers aim to overcome the limitations of current cancer treatments and pave the way for more effective and personalized therapies. The hTERT-Targeted Library, combined with ongoing research efforts, offers hope for transforming the landscape of cancer therapy and improving patient outcomes in the future.