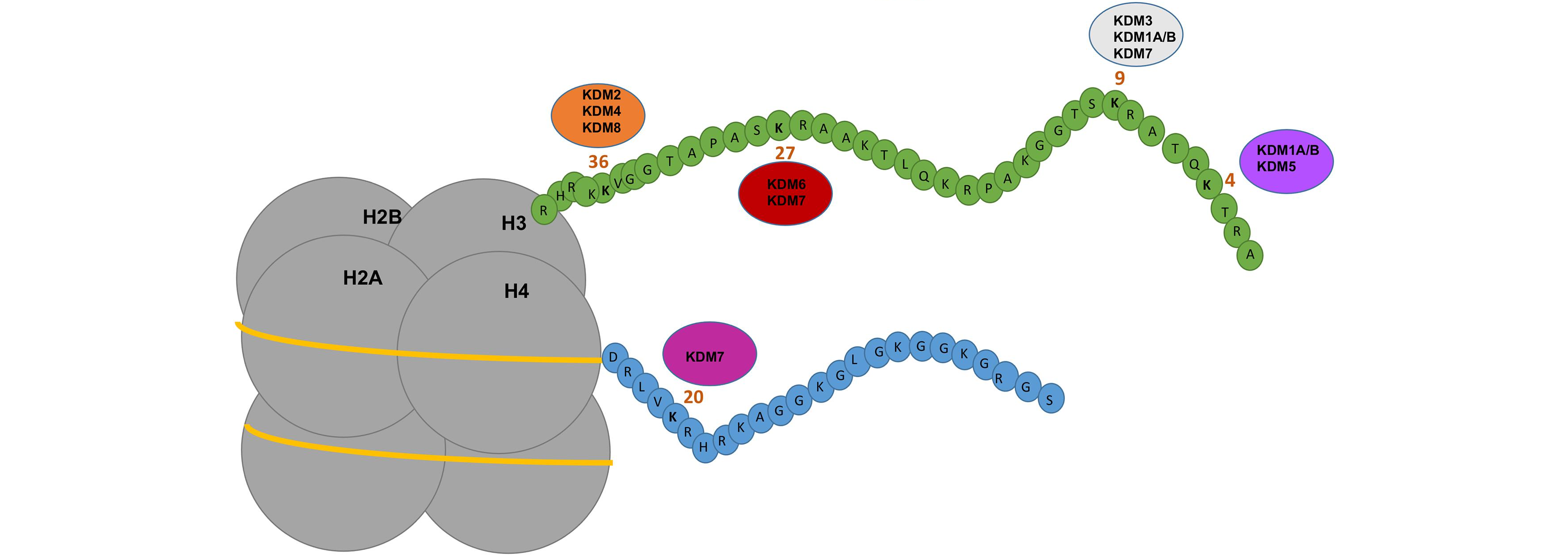
Histone methylation is an essential epigenetic modification that plays a crucial role in gene expression regulation and cellular processes. Lysine-specific histone demethylases (KDMs) are enzymes responsible for the removal of methyl groups from lysine residues in histone proteins, thereby modulating chromatin structure and gene transcription. Dysregulation of KDM activity has been implicated in various diseases, including cancer, developmental disorders, and neurodegenerative conditions. To unlock the therapeutic potential of KDM modulation, scientists have developed the Lysine-specific histone demethylases (KDM) Library – a collection of small molecules designed to selectively target and modulate the activity of KDMs.
The development of a KDM Library involves several key steps:
Structural insights and target validation:
Understanding the structure and function of KDMs is crucial for rational drug design.
Structural biology techniques, such as X-ray crystallography and cryo-electron microscopy, provide insights into the three-dimensional structure of KDMs and their active sites.
Target validation experiments help confirm the role of specific KDM isoforms in disease processes and assess their potential as therapeutic targets.
Rational drug design and virtual screening:
Utilizing the structural insights, computational techniques such as molecular docking and structure-based drug design can guide the design of small molecules that interact with the active site of KDMs.
Virtual screening of large compound libraries or fragment-based approaches can help identify initial hits or lead compounds that show potential for KDM inhibition.
Optimization and selectivity:
Lead compounds from virtual screening undergo optimization to improve their potency, selectivity, and drug-like properties.
Medicinal chemistry and structure-activity relationship (SAR) studies aim to modify the chemical structure of the compounds and optimize their pharmacokinetic and pharmacodynamic properties.
Selectivity assays are performed to ensure that the compounds primarily target specific KDM isoforms implicated in the disease being targeted while minimizing off-target effects.
High-throughput screening (HTS):
HTS approaches can be used to efficiently screen a large number of compounds from the KDM Library for their inhibitory activity against different KDM isoforms.
Assays measuring KDM activity and histone methylation levels can help identify potent and selective KDM inhibitors.
Cellular and in vivo validation:
Promising KDM inhibitors identified from HTS require further validation in cell-based assays and animal models.
Cellular assays can assess the impact of these inhibitors on gene expression, cellular differentiation, and disease-related processes.
Animal models can provide insights into the efficacy, pharmacokinetics, and potential side effects of the KDM inhibitors.
Combination therapies:
KDM inhibitors can also be explored in combination with other epigenetic modulators or traditional therapeutic approaches to enhance treatment outcomes.
Identifying synergistic combinations through screening the KDM Library with compounds targeting other epigenetic enzymes or signaling pathways can lead to new therapeutic strategies.
The development of the Lysine-specific histone demethylases (KDM) Library opens up new possibilities for epigenetic drug discovery. Targeting KDMs with small molecules allows for precise modulation of histone methylation, providing a potential strategy for reversing aberrant gene expression patterns associated with diseases. However, it is important to note that translating discoveries from the KDM Library into clinically approved drugs involves extensive optimization, validation, and clinical trials to ensure safety and efficacy.
In conclusion, the KDM Library serves as a valuable resource for researchers in their quest to develop selective and potent KDM inhibitors. By targeting KDMs, researchers can explore novel therapeutic avenues and gain deeper insights into the dynamic regulation of gene expression and epigenetic modifications. Continuing advancements in the field of epigenetic drug discovery and the expansion of the KDM Library hold the promise of transforming the treatment landscape for various diseases.