Protein-protein interactions (PPIs) plays a critical role in various cellular processes and have emerged as lucrative targets for drug development. The complexity and diversity of PPI interfaces pose significant challenges in discovering small molecule inhibitors capable of perturbing their function. Tripeptide mimetics offer a novel approach to developing PPI inhibitors, providing scaffolds through which one can modulate PPIs. In this article, we will explore the concept of Tripeptide mimetics and their importance in PPI drug discovery.
Tripeptide Mimetics Explained:
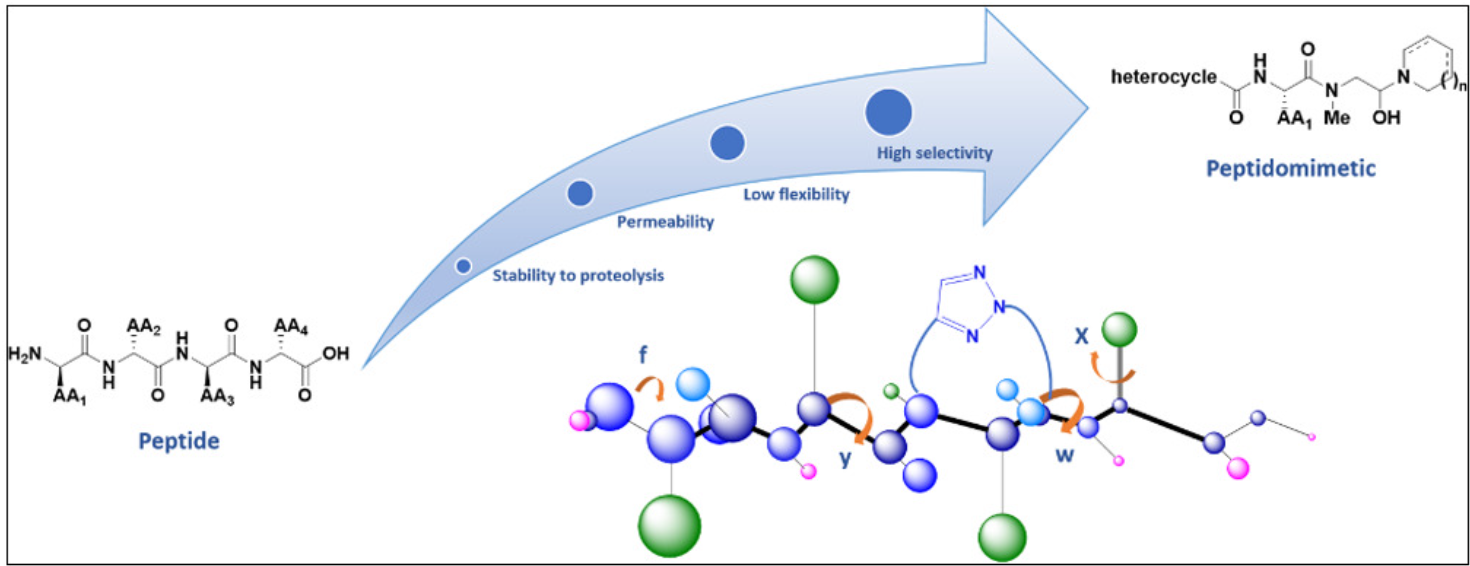
A tripeptide mimetic is a small molecule that mimics the structure and function of a tripeptide. Tripeptides often represent key functional domains in PPIs and mimicry of this domain significantly facilitates inhibitor discovery. Tripeptide mimetics can be designed to bind to the target PPI, blocking the interaction and preventing downstream cellular processes while being potent and specific in inhibiting the PPI.
Tripeptide mimetics provide several benefits over traditional small molecule PPI inhibitors. They are more stable than peptides, have better pharmacokinetic profiles, and interact with a broader range of PPI interfaces. They are easier to synthesize than peptides and allow the incorporation of diverse chemical functionalities, enabling a broader exploration of chemical space.
Need for Tripeptide Mimetics in Drug Development:
PPIs have historically been challenging to target with small molecules due to their large interface areas, lack of druggability, and the prevalence of shallow interfaces, which hinder the development of PPI inhibitors that sufficiently disrupt the interaction. The lack of small molecular inhibitors for PPIs creates a need for new strategies, such as tripeptide mimetics, to target these vital classes of protein interactions.
One of the key challenges in PPI-targeted drug development is ensuring selectivity. Tripeptide mimetics can be used to target specific interfaces with high accuracy, preventing off-target binding. Their ability to mimic key amino acid residues responsible for the interaction allows for selective targeting of the PPI of interest while maintaining specificity over other proteins.
Tripeptide mimetics have shown significant promise in various biomedical applications, including cancer, virus infections, and autoimmune diseases. In cancer, for instance, oncogenic PPIs are often overexpressed or selectively activated in tumor cells, offering a potential avenue for inhibiting their function with tripeptide mimetics. Virus infections often rely on PPIs to enter or exit host cells, and the disruption of these interactions can prevent viral replication, providing an innovative approach to antiviral drug development. Autoimmune diseases typically feature PPIs central to the pathogenic process, which tripeptide mimetics could discover roles for, resulting in novel therapeutic targets.
Applications of Tripeptide Mimetics:
Tripeptide mimetics have been used in several studies to inhibit PPIs responsible for pathological processes, thereby presenting themselves as therapeutic targets. For instance, a study employs a trimeric peptide mimic for the HIV gp41 fusion peptide, resulting in the inhibition of virus entry. Additionally, the DEPTOR-mTOR interaction is critical to several cellular processes, and the inhibition of PPIs may disrupt downstream signaling, providing a potential avenue for therapeutic intervention.
Tripeptide mimetics have also been applied to inhibit PPIs between proteins and nucleic acids. For instance, a tripeptide mimetic targeting the hnRNP A1 protein, an mRNA processing regulator, leads to the inhibition of the PPI with RNA, thereby providing an opportunity to intervene against diseases with dysregulated A1-RNA interactions.
Challenges and Future Directions:
Despite the potential of Tripeptide mimetics for PPI-targeted drug discovery, several challenges hinder their widespread application. Improvements in the computational tools used in the design of Tripeptide mimetics, especially in predicting their activity and selectivity, are critical to their success.