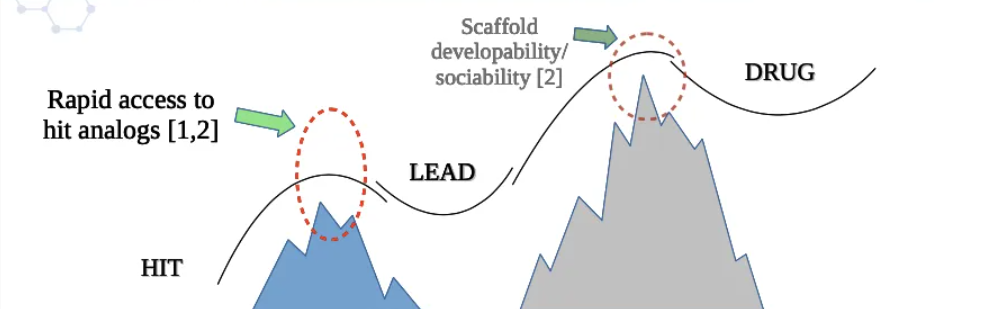
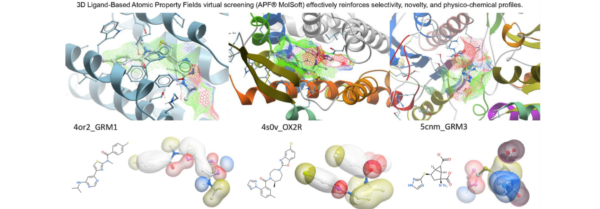
The concept of a Fast Follow-Up SAR Diverse Screening Library has gained significant attention in the field of drug discovery. This library is designed to rapidly identify and optimize lead compounds by incorporating a diverse collection of structurally distinct molecules that possess desired drug-like properties. It serves as a valuable resource for researchers attempting to expedite the hit-to-lead optimization process and accelerate the development of potential therapeutic agents.
The Fast Follow-Up SAR Diverse Screening Library is composed of a wide range of compounds, carefully selected to capture structural diversity and chemical space. These compounds are typically derived from various sources, including commercially available small molecules, in-house chemical libraries, natural products, and computationally designed molecules. The diversity in chemical structures allows researchers to explore a broad spectrum of potential hits and leads, enabling them to identify the most promising compounds for further development[1][2].
The primary goal of this library is to rapidly identify lead compounds through high-throughput screening campaigns. By screening a large number of compounds against a specific target, researchers aim to identify molecules that demonstrate the desired biological activity or binding affinity. The diverse nature of the library increases the likelihood of finding hits that cover a wide range of chemical space, enhancing the chances of identifying structurally diverse lead compounds[3].
Once hits are identified, the follow-up process involves structure-activity relationship (SAR) studies. SAR involves synthesizing analogs and derivatives of the hit compounds in order to study the relationship between the structure of the compounds and their biological activity. The Fast Follow-Up SAR Diverse Screening Library plays a crucial role in this process by providing a collection of diverse compounds that can be utilized to generate SAR data quickly. By exploring a wide range of chemical modifications and substitutions, researchers can rapidly optimize compound potency, selectivity, and other desirable pharmacological properties[4].
The advantage of a Fast Follow-Up SAR Diverse Screening Library lies in its ability to accelerate the hit-to-lead optimization process. By incorporating diverse compounds in the library, researchers can explore a broader range of chemical space in a shorter timeframe. This approach increases the probability of finding structurally distinct lead compounds with improved properties[5].
Furthermore, the library allows researchers to gain important insights into the structure-activity relationships of the hit compounds. By synthesizing analogs and derivatives of the initial hits, researchers can quickly assess the impact of specific modifications on compound activity, thereby guiding the lead optimization process more efficiently. This iterative process rapidly narrows down the chemical space, leading to the identification of optimized lead compounds for further development[6].
In summary, the Fast Follow-Up SAR Diverse Screening Library is an invaluable tool in the hit-to-lead optimization process. By incorporating a diverse collection of compounds, it enables researchers to rapidly identify lead compounds and optimize their potency, selectivity, and other desired properties. By exploring a wide range of chemical space and conducting structure-activity relationship studies, researchers can efficiently advance potential drug candidates to the next stage of development. The library serves as a valuable resource in accelerating the drug discovery process and ultimately contributes to the development of new therapeutic agents.
References:
Medina-Franco, J. L., Giulianotti, M. A., & Welmaker, G. S. (2014). Heterocyclic chemistry in drug discovery. Drug Discovery Today, 19(6), 737-743.
Walters, W. P., Murcko, M. A., & Murcko, M. A. (1999). Recognizing molecules with drug-like properties. Current Opinion in Chemical Biology, 3(4), 384-387.
Lovering, F., Bikker, J., & Humblet, C. (2009). Escape from Flatland: Increasing Saturation as an Approach to Improving Clinical Success. Journal of Medicinal Chemistry, 52(21), 6752-6756.
Lipinski, C. A. (2004). Lead- and drug-like compounds: the rule-of-five revolution. Drug Discovery Today: Technologies, 1(4), 337-341.
Van de Waterbeemd, H., & Gifford, E. (2003). ADMET in silico modelling: towards prediction paradise?. Nature Reviews Drug Discovery, 2(3), 192-204.
Congreve, M., Carr, R., Murray, C., & Jhoti, H. (2003). A “Rule of Three” for Fragment-Based Lead Discovery? Drug Discovery Today, 8(19), 876-877.