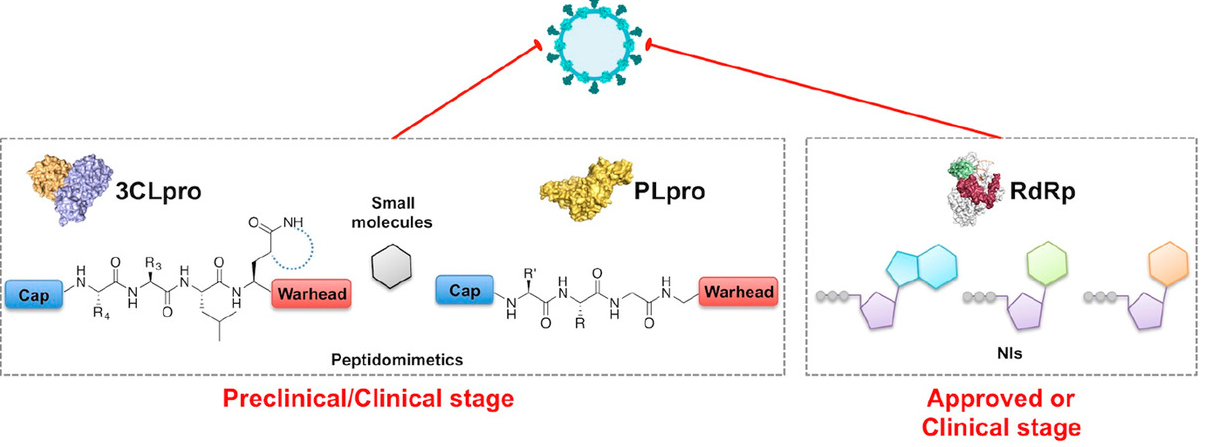
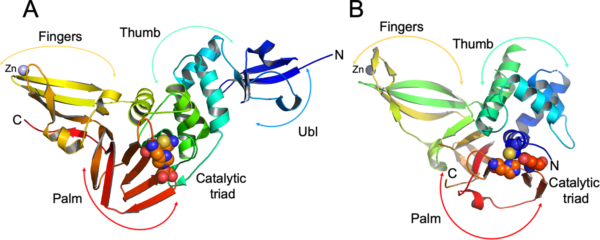
The 3CLpro Library plays a crucial role in the regulation of viral replication, especially in the context of viral infections caused by positive-sense RNA viruses. 3CLpro, also known as the main protease or Mpro, is an enzyme essential for the replication of these viruses. It is responsible for the cleavage of polyproteins that are translated from the viral RNA, generating functional viral proteins necessary for viral replication and assembly.
The 3CLpro Library comprises a collection of small molecule inhibitors designed to specifically target the catalytic activity of 3CLpro. These inhibitors are designed through structure-based drug design techniques, taking advantage of the three-dimensional structure of the protease to guide the design and optimization of potent and selective inhibitors.
By targeting the catalytic activity of 3CLpro, the library aims to disrupt the proteolytic processing of viral polyproteins, ultimately inhibiting viral replication. Inhibition of 3CLpro prevents the generation of essential viral proteins and impairs the viral life cycle, reducing the overall viral load within the host.
Through the use of the 3CLpro Library, researchers can screen and identify potential lead compounds with inhibitory activity against 3CLpro. These compounds can serve as starting points for further optimization and development of antiviral therapeutics. By selectively targeting the viral protease, these inhibitors offer the potential for high specificity, minimizing off-target effects and reducing the likelihood of viral resistance.
The 3CLpro Library addresses an urgent need in antiviral drug discovery, particularly in the context of emerging viral diseases. Positive-sense RNA viruses, such as coronaviruses, are known to rely heavily on the activity of 3CLpro for their replication. Inhibiting this crucial enzyme provides an avenue for therapeutic intervention, potentially controlling viral replication and mitigating the severity of viral infections.
The development and optimization of the 3CLpro Library require a multidisciplinary approach, involving medicinal chemists, structural biologists, virologists, and computational scientists. By combining the knowledge of the enzyme’s structure, substrate specificity, and catalytic mechanism, researchers can design inhibitors that effectively bind to and inhibit the activity of 3CLpro.
In conclusion, the 3CLpro Library plays a vital role in the regulation of viral replication, specifically targeting the activity of the main protease. By selectively inhibiting this enzyme, the library aims to disrupt critical proteolytic processes required for viral replication. The 3CLpro Library represents a valuable resource for identifying potential antiviral compounds and advancing drug discovery efforts in the fight against viral infections caused by positive-sense RNA viruses.